AMOEBA: Cancellation of the issue of the 8th optional tranche of OCAs and signature of a support contract with Redbridge Debt and Treasury Advisory
Lyon (France), January 27, 2023 – 5h45 pm – AMOÉBA (FR0011051598 – ALMIB), producer of a biological biocide capable of eliminating bacterial risk in water and human wounds, and of a biocontrol product for plant protection, still in development phase, announces that it has terminated its convertible bond issue contract with Nice & Green SA and waived the issue of the 8th optional tranche of 80 OCAs as initially planned in the contract[i]. In order to adapt its financial strategy to its new industrial transformation challenges, Amoéba has concluded a contract with Redbridge Debt and Treasury Advisory to assist it in its search for financing.
As a reminder, Amoéba’s ambition is to build a production plant dedicated to biocontrol products, which could produce 40 tons of active substance per year, the equivalent of 100,000 hectares treated. This production site should be operational by early 2025 in order to start marketing its biocontrol products as soon as marketing authorisations are obtained in Europe and the United States. In order to finance this production site and to continue its operational and research activities over the next 3 years, the company estimates its total financial needs at €45 million. Amoéba has selected the independent advisory firm Redbridge Debt & Treasury Advisory to assist it in optimising its debt structure and putting in place the necessary financing.
This support should be carried out in 2 phases:
1) Search for the optimal financing strategy(ies) for Amoéba
2) Support in the structuring, promotion and execution of its financing operations until the 45 million euro envelope is obtained.
“Thanks to the financing received from Nice & Green over the last 3 years, Amoéba has been able to pursue its research and development efforts on the biocontrol application and is now entering a new phase of its industrial and commercial development. By relying on the experience of Redbridge Debt & Treasury Advisory, we hope to optimise our financing strategy and find the most suitable financing for our development“, says Fabrice PLASSON, Chairman and CEO of Amoéba.
[i] See press release of December 21, 2020
US EPA approves Amoeba’s biocide for use in closed cooling systems
Lyon (France), Décember 20th, 2022 – 17h45 – AMOÉBA (FR0011051598 – ALMIB), producer of a biological biocide capable of eliminating bacterial risk in water and human wounds, and of a biocontrol product for plant protection, still in development phase, informs that the US Environmental Protection Agency (US EPA) has approved the use of the Willaertia magna C2c Maky as an active substance for use cooling systems.
As a result of the EPA’s favorable pre-decision (see Press Release dated August 10th, 2022) and the public consultation required for any new active substance, completed on December 3rd, 2022, the amoeba Willaertia magna C2c Maky and the BIOMEBA products containing it are now approved in the United States for a biocidal use in closed cooling systems for the control of microbial slime (bioslime), microbially induced corrosion and general microbial flora.
“After more than 10 years of research and development, Amoéba is the very first company in the world to validate a biological biocide in the treatment of bacterial risk in water! This is a major success that the company will tend to develop over the next few years”, says Fabrice Plasson, CEO of Amoéba.
Besides, to continue the strategic alignment of the company, Amoéba has initiated the withdrawal of the biocidal active substance application in Europe, as was done in Canada (see Press Release dated October 19th, 2022).
AMOEBA announces the issuance of the seventh tranche of 40 bonds convertible into shares
Chassieu (France), December 14, 2022– 5.45 pm – AMOEBA (FR0011051598 – ALMIB), producer of a biological biocide capable of eliminating bacterial risk in water and human wounds, and a biocontrol product for plant protection, still in a development phase, announces the issuance of the seventh tranche of bonds convertible into shares (“OCAs”) of its bond financing with incentive program, namely 40 OCAs numbered from 361 to 400 fully issued to Nice & Green S.A.
This issue is part of the agreement entered into with Nice & Green S.A. on December 16, 2020 with a view to setting up a bond financing with a profit-sharing program through the issuance of 480 OCAs with a nominal value of EUR 50,000 each, representing a total nominal amount of the bond issue of EUR 24,000,000 (the “Issuance Agreement”).1
The Chairman and Chief Executive Officer of the Company, using the sub-delegation granted to him by the Board of Directors at its meeting on June 24, 2021, decided to issue on December 13, 2022, 40 OCAs numbered from 361 to 400 to the benefit of Nice & Green S.A. corresponding to the seventh tranche of the bond financing.
As provided for in the issuance agreement, these OCAs were fully subscribed at a price equal to 96% of their nominal value, representing a seventh tranche of OCAs for a total net amount of EUR 1,920,000.
As a reminder, the Company maintains on its website a monitoring table of the OCAs and the number of Amoéba shares in circulation (see Investors section / Regulatories information / Other information).
As an indication, the theoretical impact of the issue of this seventh tranche of OCAs is presented in the tables below in accordance with the OCA conversion formulas on the basis of 92% of the lowest volume-weighted average trading price of the Amoéba share at closing (as published by Bloomberg) over the six (6) trading days immediately preceding December 13, 2022, namely 0.8238 euros.
– Impact of the issue on the share of shareholders’ equity per share (calculation based on Amoéba’s shareholders’ equity as at june 30, 2022, prepared in accordance with International Financial Reporting Standards (IFRS) adjusted for capital increases completed up to December 13, 2022 i. e. 10,572,011 euros and the number of shares comprising the Company’s share capital as at December 13, 2022, i. e. 46,309,880 shares) :
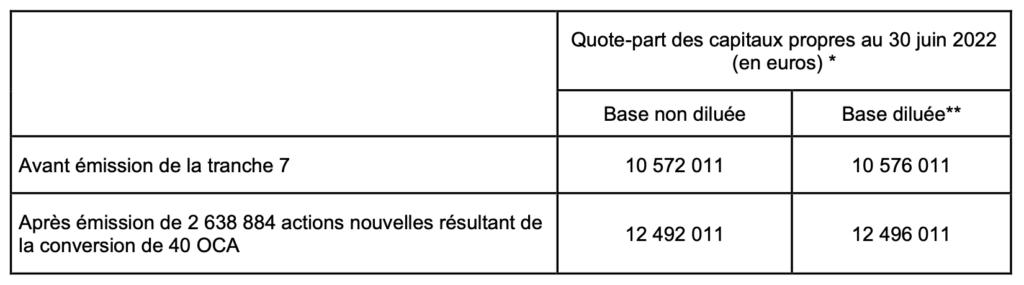
(*) amount of shareholders’ equity at 30 june 2022 prepared in accordance with IFRS international financial standards and adjusted for capital increases completed until December 13, 2022
(**) assuming the full exercise of the share subscription warrants issued and allocated by Amoéba, exercisable or not, giving the right to subscribe for 200,000 new shares
– Impact of the issue on the participation of a shareholder holding 1% of Amoéba’s share capital prior to the issue of the seventh tranche (calculation based on the number of shares comprising Amoéba’s share capital as at December 13, 2022, i.e. 46,309,880 shares) :
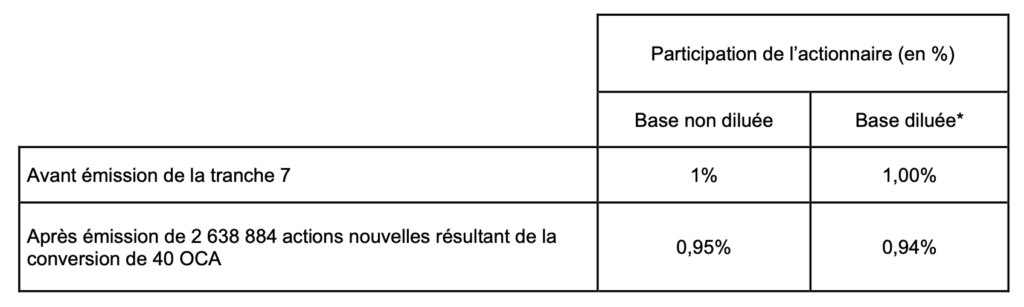
(*) assuming the full exercise of the share subscription warrants issued and allocated by Amoéba, exercisable or not, giving the right to subscribe for 200,000 new shares
US EPA approves Amoeba’s biocontrol solution for agricultural use
Lyon (France), November 3, 2022 – 8.45 am- AMOÉBA (FR0011051598 – ALMIB), producer of a biological biocide capable of eliminating bacterial risk in water and human wounds, and of a biocontrol product for plant protection, still in development phase, informs that the US Environmental Protection Agency (US EPA) has approved the use of the lysate of Willaertia magna C2c Maky as an active substance for use in plant protection.
As a result of the EPA’s favorable pre-decision (see Press Release dated September 29th, 2022), the lysate of the amoeba Willaertia magna C2c Maky is now approved for use in the United States for the control of fungal plant diseases in agriculture.
In accordance with the pre-decision, the US EPA has confirmed the exemption of Willaertia magna C2c Maky lysate from maximum residue limits (see Press Release dated October 12th, 2022) and exemption of pre-harvest intervals when applied in accordance with instructions for use and good agricultural practices.
Formulations (products) containing Willaertia magna C2c Maky lysate will be subject to an application for marketing authorization to the EPA in 2023, with an approval expected in 2024.
Amoéba: a record year for the number and performance of field trials
Chassieu (France), October 20, 2022 – 5.45 pm – AMOÉBA (FR0011051598 – ALMIB), producer of a biological biocide capable of eliminating the risk in water and human wounds, and of a biocontrol product for plant protection, still in the testing phase, announces the publication of its 2022 field trial results.
With more than 120 field trials completed or underway in Europe, the United States, Brazil, Costa Rica and Asia, the winter 2021/summer 2022 field trial campaign is the largest ever undertaken by Amoéba.
The main objectives of these trials conducted by independent external service providers in small plots under GEP (Good Experimentation Practices) are to :
- Generate efficacy data for future marketing authorization applications in Europe, Brazil and California
- Evaluate the company’s formulations on new targets, in particular diseases of tropical crops, apple and certain vegetable crops.
- Conduct positioning trials in combination or in programs with other fungicides (in particular on vines, potatoes, wheat, vegetables and soybeans), foreshadowing experiments closer to practical use.
Two main formulations were tested depending on the crops: a suspension concentrate (SC) and an oil dispersion (OD).
1 – Crops / diseases already experimented in previous years
Vine / Wheat / Potato
Recent trials allowed to confirm the chosen rates, in liters per hectare (L/ha), for future marketing authorization applications and to accentuate the efforts to practically apply them (in associations or programs):
- Vine (mildew and powdery mildew): 2.5 L/ha alone – 1.25 L/ha in combination with copper
- Potato (late blight): 1.25 L/ha
- Wheat (depending on diseases): 1.25 to 2.5 L/ha
Vegetable crops
A major campaign of 27 trials was conducted in 2022 in Southern Europe and California, and is still ongoing in Spain and Italy to test the efficacy of our product on vegetable crops.
Against downy and powdery mildews on field crops (cucurbits, lettuce) the product confirmed the good results of previous years. Almost systematically, the performance was better compared to other biocontrol products.
On tomatoes, grown in open field and intended for processing, all trials this year, conducted in the heart of the main growing area in Italy, confirmed the great potential of the product. In fact, our solution was as efficient as copper against mildew, especially for the protection of fruits, in a situation of medium-high infestation. It is thus possible, in a program including 6 treatments, to replace 4 copper treatments by our solution without impacting the level of protection (up to 80% efficiency on leaves and 100% on fruits).
2 – New targets / Temperate crops
Vegetable crops under greenhouse
Against powdery mildew in greenhouse crops (tomato and cucumber), very good results have been measured for this first year of experimentation in Greece and Italy. Sometimes less efficient than the reference sulfur, the product appears systematically more efficient than the reference biocontrol products (70% efficiency on cucumber for example, against only 24% for the reference).
Apple tree
This year is also marked by the start of trials on a new target category: fruits, in particular apples. Massively treated (up to 20 treatments per year for European producers) and exposed to chemical residues, apples represent an important world market, continuously in search of biofungicides.
Two trials on scab conducted in France and Italy on lightly contaminated apple trees have demonstrated strong activity of the product (particularly on fruit), similar to copper at its highest tested dose.
This result will be confirmed in situations of more severe infestation, in this important market lacking any natural solutions to date.
Turf
The first trial against Fusarium on turf during the winter of 2021/2022 on a golf course in Italy showed 50-60% efficacy with both formulations. This performance is statistically equivalent to the one of the reference chemical fungicide.
3 – New targets / Tropical crops
The product has been tested in all major tropical markets.
Soybean
A campaign of about ten trials was conducted in Brazil in different producing states (Mato Grosso, Mato Grosso do Sul, Goias, Parana, Rio Grande do Sul). These trials targeted the main disease, Asian soybean rust, but also a series of so-called “end-of-cycle” diseases, notably target spot, septoria, cercosporiosis and powdery mildew.
Under these conditions, good efficacy was observed with both tested formulations, and this at relatively low dose rates. Even in the most contaminated trials, the product efficacy was frequently similar to that of the widely used reference fungicide (chlorothalonil).
Furthermore, when used in a mixture, the product complements well the performance of a chemical fungicide, making it a very useful combination to limit the appearance of resistant rust strains, responsible for the efficacity decrease of the latest chemical fungicides.
In one of the most infested trials, the best treatment was this combination of chemical fungicide and Amoeba suspension concentrate, statistically superior to all references and other combinations (66% efficacy against 40-45% for the other programs). A high versatility against all end-of-cycle diseases was also observed.
Our product therefore confirms its effectiveness against soybean rust, as well as its potential to be integrated into field crop treatment programs.
Banana
For the first year, three trials were conducted (Indonesia, Brazil, trial in progress in Costa Rica) against the main banana disease: black Sigatoka. In the wettest growing areas, bananas are treated all year round, once a week (52 applications per year).
The results of the first two completed trials show that the products (especially with the OD oil dispersion formulation) have the same performance as chlorothalonil (45% efficacy in one, 95% in the other), one of the most widely used fungicides on this crop. This old fungicide, banned in Europe in 2019, will probably be banned in the medium term in many banana-producing countries.
Furthermore, an in-vitro test carried out by a specialized laboratory in Costa Rica has just demonstrated that amoeba formulations inhibit the germination of Mycosphaerella fijiensis spores, which is a key advantage on this crop where spores are permanently present in the environment.
This result confirms this mode of action, which has already been observed on many pathogens (notably downy mildew on grapes and soybean rust).
There is therefore great potential in this major crop where all the operators are looking for non-chemical, alternative or complementary solutions to preserve the environment, reduce residues on fruit and create associations with the best chemical fungicides in order to limit the risk of emerging resistant strains.
The number of trials will now be increased, in order to specify the practical rate and to start working on the integration of the product in the annual treatment programs.
4 – Conclusions
Four years of field experimentation, with more than 300 trials conducted by Amoéba in many countries, has contributed to the acquisition of a solid knowledge on Willaertia magna C2c Maky lysate-based products.
The broad spectrum, the ability to control many diseases on specialized crops as well as on field crops, in temperate climate and also in tropical areas, the higher level of performance compared to biofungicides available on the market, allow to consider a positioning of Amoeba’s products as an alternative or as a complement to chemical fungicides, and in particular as a direct substitute of the two most important contact fungicides used in the world, mancozeb and chlorothalonil (both already banned in Europe), on a certain number of crops.
Amoeba withdraws its biocide file in Canada to focus its resources on strategic priorities
Lyon (France), October 19, 2022 – 17h45 – AMOÉBA (FR0011051598 – ALMIB), producer of a biological biocide capable of eliminating bacterial risk in water and human wounds, and of a biocontrol product for plant protection, still in development phase, announces that it will not pursue the application for its living amoeba-based biocide in Canada.
Considering the high regulatory risk of this application, which mobilizes human and financial resources, Amoeba has decided to definitively withdraw the application for registration in Canada and to focus its resources on low regulatory risk applications, mainly the biocontrol application which has received positive evaluations in Europe and in the United States so far (see Press Releases of April 25, 2022 and September 29, 2022 respectively) and the biocidal application in closed cooling towers in the United States (see Press Release of August 10, 2022).
Amoeba is also pursuing its research and development of other uses of the amoeba, in its non-living lysed form, which presents a much lower regulatory risk than the living form, and therefore a high chance of success.
Amoéba: publication of a scientific article concerning the efficacy of its biocontrol products to fight potato late blight
Chassieu (France), October 18 2022 – 5.45 pm – AMOÉBA (FR0011051598 – ALMIB), producer of a biological biocide capable of eliminating the risk in water and human wounds, and of a biocontrol product for plant protection, still in the testing phase, announces the publication of a second scientific article on its biocontrol application (https://www.mdpi.com/2223-7747/11/20/2756/pdf) in the special issue « Plant Bioprotection » de Plants, journal of the MDPI Group, peer-reviewed.
This paper presents, for the first time to the international scientific community, the efficacy of the biocontrol products based on the lysate of the amoeba Willaertia magna C2c Maky on potato late blight. Scientific data demonstrating the dual mode of action of the lysate to control potato late blight are presented:
– the indirect effect via the stimulation of the plant’s natural defences
– the direct anti-germinative effect against the pathogen, Phytophtora infestans, responsible for potato late blight.
The data, collected in trials carried out by independent service providers, that have demonstrated efficiency in greenhouses and in the field (2020 and 2021 seasons), are also published. They show that lysate of the amoeba Willaertia magna C2c Maky, a product of natural origin, protects plant without additional treatment up to 77% when the disease was low (28% of the surface of the untreated plants affected) and up to 49% when the untreated plants were destroyed at 100%. A yield increase was also obtained with up to 30% more tubers.
« This second scientific paper in the frame of plant protection area, dealing with the efficacy of our biocontrol solution against potato late blight, is part of an ongoing effort to increase scientific knowledge on the amoeba Willaertia magna C2c Maky. It confirms its strong potential as a plant protection agent. We are continuing R&D operations and tests on other crops, the results of which will also be the subject of new publications,” says Dr Sandrine Troussieux, chief scientific officer at Amoéba.
Amoéba informs that the US EPA approves tolerance exemption for the biocontrol active substance on foodstuffs
Lyon (France), October 12th, 2022 – 1h15pm – AMOÉBA (FR0011051598 – ALMIB), producer of a biological biocide capable of eliminating bacterial risk in water and human wounds, and of a biocontrol product for plant protection, still in development phase, informs that the U.S. Environmental Protection Agency (US EPA) has approved the tolerance exemption for the biocontrol active substance on foodstuffs.
Following its positive pre-decision (see Press Release of September 29, 2022), the EPA has approved the exemption of a maximum permissible level (tolerance exemption) for the lysate of Willaertia magna C2c Maky, corresponding to the regulatory threshold of pesticide residue concentration, above which the marketing of a food product is no longer allowed.
The decision of the US EPA can be seen on the federal register:
https://www.federalregister.gov/documents/2022/10/12/2022-22045/lysate-of-willaertia-magna- c2c-maky-exemption-from-the-requirement-of-a-tolerance
The Company is still awaiting the final decision on the authorization of the biocontrol active substance by the EPA, which is expected before the end of October.
Amoéba announces the publication of its Interim Financial Report for 2022.
Chassieu, October 3, 2022, 5.45 pm, AMOEBA (FR0011051598-ALMIB) – producer of a biological biocide capable of eliminating the risk in water and human wounds, and of a biocontrol product for plant protection, still in the testing phase, announces today that it has published its Interim Financial Report for 2022.
AMOEBA’s shareholders are informed that the interim financial report for the 2022 financial year (ending on December 31st, 2022), filed today with the Autorité des Marchés Financiers (AMF), can be found at the company website: https://amoeba-nature.com/en/investor/financial-documents/.
This report includes:
• Interim Activity Report
• Condensed Interim Financial Statements
• Certification of the person responsible for the interim financial report
• Statutory Auditors’ Review Report on the interim financial information
Amoéba announces its half-year results for 2022
Chassieu (France), September 29, 2022 – 6:00 pm – AMOÉBA (FR0011051598 – ALMIB), producer of a biological biocide capable of eliminating bacterial risk in water and human wounds, and of a biocontrol product for plant protection, still in the development phase, today announced its half- yearly results for 2022.
The Board of Directors, which met on 29 September 2022, approved the Company’s consolidated accounts for the first half of 2022.
The Statutory Auditor has carried out a limited review at the Company’s request of the consolidated financial statements for the six months ended 30 June 2022 and has not identified any material misstatements that would call into question their conformity.
The half-year report is being issued and will be available on the company’s website (www.amoeba- nature.com) in the next few days.
Operational results in line with 2021 and financial debt restructuring
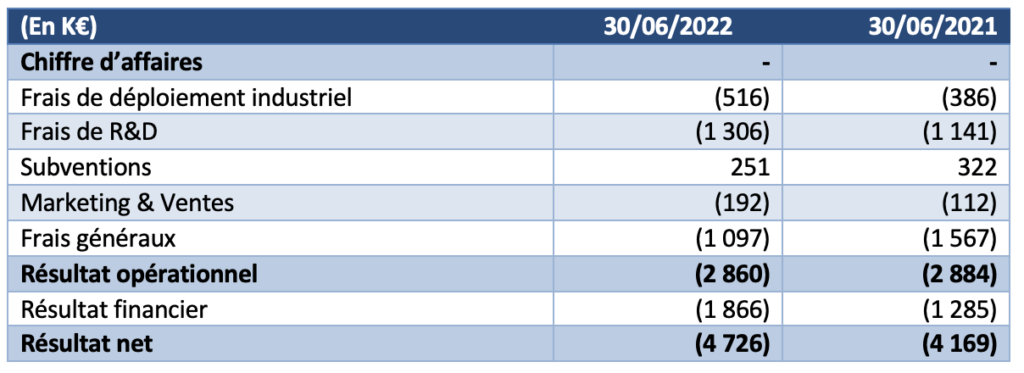
The operating result for the six months to 30 June 2022 was a loss of K€2,860, compared with a loss of K€2,884 in the first half of 2021. No significant impairment was recorded during the periods presented.
- The company did not generate any revenue during the first half of 2022.
- Industrial deployment costs were €130k higher than the previous year.
- Research and Development expenses net of grants were €1,055k, compared to €819k in H12021.
- Marketing and sales expenses are up 71% on the previous year (€192k at 30 June 2022 versus €112k at 30 June 2021
- General and administrative expenses were down at 30 June 2022 to €1,097k, or -30% compared to the previous year.
- The financial result mainly includes interest expenses related to bank loans of €1,549k (compared to €1,434k in the first half of 2021) and changes in the fair value of financial derivatives of €335k (compared to €154k in 2021).
The net result for the first half of the year is therefore -€4,726k.The Company’s cash position at 30 June 2022 was €2,752k compared to €7,274k at 31 December 2021.
The decrease in cash is explained by :• cash flow from operations of – €2,682k;
• cash flow from investment activities of -€40k;
• cash flows from financing activities of – €1,802k, mainly due to the issue of bonds for €5,760kin the first half of 2022 and the full repayment of the EIB loan for €6,070k.As at 30 June 2022, the Company’s shareholders’ equity amounted to €3.7m compared to €0.2m as at 31 December 2021.The Company’s financial debt amounted to €4.8m. It is mainly made up of the OCAPI loan (€4.3 million) and debts linked to rental obligations (€0.4 million), the EIB loan having been fully repaid on 30 June 2022.
A first semester mainly marked by the progress of regulatory marketing files, the continued development of the biocontrol application and the early repayment of the EIB loanDuring the first half of 2022, Amoéba focused on the following main areas:
1. Follow-up of the marketing authorisation applications for the biocontrol and biocideapplications
used in accordance with good plant protection practice and under realistic conditions of use.
• Biocidal application
On 03 May 2022, the Company announced that the MCCAA (Malta Competition and Consumer Affairs Authority), the competent authority of the reporting Member State (Malta) evaluating the application for approval of the biocidal active substance “Willaertia magna C2c Maky”, recommended its non-approval for biocidal use in cooling towers in Europe.
On the basis of the application dossier for approval of the biocidal active substance “Willaertia magna C2c Maky”, the Maltese authority concluded in its draft report that the active substance is not likely to meet the approval criteria, considering that the innate efficacy has not been sufficiently demonstrated and that a Trojan horse effect cannot be excluded under realistic conditions of use. However, a few weeks later, the US EPA issued a favourable pre-notification for the use of the amoeba Willaertia Magna C2c Maky in closed cooling systems (see below “Recent developments and prospects”) in the United States.
2.Further development of the biocontrol application and launch of a new massive field trial campaign for 2022
In the first half of 2022, the company has started a new field trial campaign for its biocontrol product, focusing on :
- Trials for the European marketing authorisation of the selected formulation on grapevine downy mildew
- After the excellent results obtained in 2021, the intensification of the powdery mildew programme on vines
- Intensification of the programme against mildew and powdery mildew on market garden crops in order to prepare future MA applications
- Continuation of the cereals programme, in particular against rusts, septoria and fusarium head blight.
- Trials on new targets: in particular apple scab, a major subject, following promising results obtained in climatic chambers.
- Continued evaluation against soybean rust and coffee rust FinancingThe Company announced the issuance of the fourth and fifth tranches of 60 bonds convertible into shares in the framework of its bond financing with an incentive programme concluded with Nice & Green.In addition, Amoéba finalised the restructuring of its debt by prepaying the entire EIB loan.
3.Financing
The Company announced the issuance of the fourth and fifth tranches of 60 bonds convertible into shares in the framework of its bond financing with an incentive programme concluded with Nice & Green.In addition, Amoéba finalised the restructuring of its debt by prepaying the entire EIB loan.
4.Changes in governance
The General Meeting of Shareholders of 24 May 2022 ratified the appointment as directors of Mr Philippe DUJARDIN to replace Mr Pascal REBER, who resigned and Mrs Sylvie GUINARD, replacing Mrs Claudine VERMOT-DESROCHES, who has resigned.
Mr Philippe DUJARDIN and Ms Sylvie GUINARD will hold office for the remainder of their predecessors’ term of office, i.e. until the end of the Ordinary General Meeting of shareholders to be held in 2023 to approve the financial statements for the year ending 31 December 2022.
In addition, the General Meeting of Shareholders of 24 May 2022 ratified the appointment of Mr Pascal REBER as Censeur.
Mr Pascal REBER will exercise the said functions for a period of three (3) years, i.e. until the end of the Ordinary General Meeting of Shareholders to be held in 2024 to approve the accounts for the financial year ending 31 December 2023.
Impact of the COVID-19 health crisis and the war in Ukraine on the accounts at 30 June 2022
At the date of this half-yearly report, the Company considers that its activities have not been significantly impacted by the health crisis.
The production of the active substance necessary to carry out the field tests could continue under normal operating conditions. The health crisis had no impact on the preparation and monitoring of current regulatory dossiers. The Company made limited use of the partial activity mechanism and did not request an EMP.
The Company does not yet market its products and does not recognise any significant turnover to date. The Covid-19 crisis had little impact on its income statement.
The Company has no activities in Russia or Ukraine. However, the Company’s activities could be impacted by the direct or indirect consequences of the conflict, which it is not possible to quantify precisely at this time.
Recent developments and prospects
Following the US EPA’s preliminary favourable decision on the use of the amoeba Willaertia Magna C2c Maky in closed cooling systems (see press release of 10 August 2022), Amoeba is currently refining its market analysis on this restricted type of system in order to evaluate its commercialization potential.
On 29 September 2022, the company has received from the United States Environmental Protection Agency (US EPA) a positive pre-decisional determination following its assessment of the application dossier for the use of the Lysate of Willaertia magna C2c Maky as a biocontrol active ingredient (biopesticide) in agriculture. The US EPA has concluded that the Lysate of Willaertia magna C2c Maky has a low toxic profile for human health and the environment, and that its “mode of action contributes to its attractiveness as viable alternative to conventional pesticides making it a valuable addition to the pesticide tool kit”. Therefore, the EPA is proposing to grant the unconditional registration of lysate of Willaertia magna C2c Maky as a new active ingredient and supports a pesticidal food use and non- food use.
The Company is pursuing its project to set up its first biocontrol plant. This plant would be located in France and should be operational in 2024 to satisfy the start of the marketing of biocontrol products. It should represent an estimated investment of between 15 and 17 million euros for which Amoéba will have to seek new financing from the 4th quarter of 2022.
At the date of closing of the accounts, the Company has sufficient net working capital to meet its obligations and cash requirements over the next twelve months, as the Company believes it can meet its commitments.
