Regulatory timetable for Europe’s biocontrol application on track
AMOEBA: Regulatory timetable for Europe’s biocontrol application on track
Chassieu (France), July 13th, 2023 – 08h30 – AMOÉBA (FR0011051598 – ALMIB) an industrial biotech in pre-commercialization* specialized in the treatment of microbiological risk, developing a biocontrol agent for the treatment of plants in agriculture and a biological biocide for the treatment of industrial water, confirms that the regulatory timetable for the biocontrol active substance application is in line with the Company’s forecasts.
The EFSA, the European Food Safety Agency, mentions on its website a Risk Assessment Deadline on July 19, 2023. As this date was assigned by default when the peer-review of dossier was initiated in December 2022, it does not take into account any delay by the authorities.
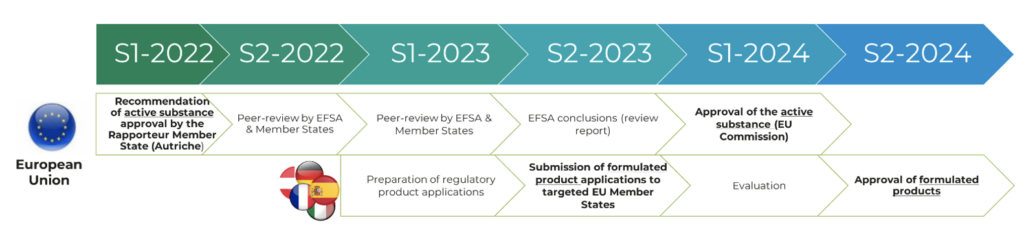
The Company had anticipated this possible delay in its forecasts: the regulatory timetable for its biocontrol applications in Europe, as published in the Universal Registration Document 2022 filed with the AMF on April 18, 2023 under the number D23-0296 and available on the Company’s website, is still valid and is recalled below:
- Risk assessment phase ending with EFSA’s peer-review report containing its conclusions: expected to be finalized in the second half of 2023.
- Risk management phase ending with the publication of the implementing regulation containing the European Commission’s decision in the Official Journal of the EU: expected to be finalized in the first half of 2024.
As applications for approval of formulated products can be submitted before definitive approval of the active substance, the forecast timetable for formulated products is also in line with the Company’s forecasts.