Amoéba: a first half-year mainly marked by the development of the biocontrol plant project
Chassieu (France), October 5, 2023 – 18:00 CET – AMOÉBA (FR0011051598 – ALMIB), which is producing a biological biocide capable of eliminating the bacterial risk in water and a biocontrol product for plant protection, still in a development phase, is today reporting its earnings for the first half of 2023, ended June 30, 2023, as approved by the Board of Directors on October 5, 2023.
At the end of 2022, Amoeba obtained approval of its active substance for biocidal use in closed cooling systems and for biocontrol use in the United States.
As requested by the Company, the Statutory Auditors carried out a limited review of the consolidated half-year accounts at June 30, 2023 and did not identify any material misstatements that would call into question the compliance of these accounts.
The half-year report is currently being issued and will be published on the Company’s website (www.amoeba-nature.com) over the coming days.
Results that reflect the start of the industrial project and a significant reduction in the debt charges
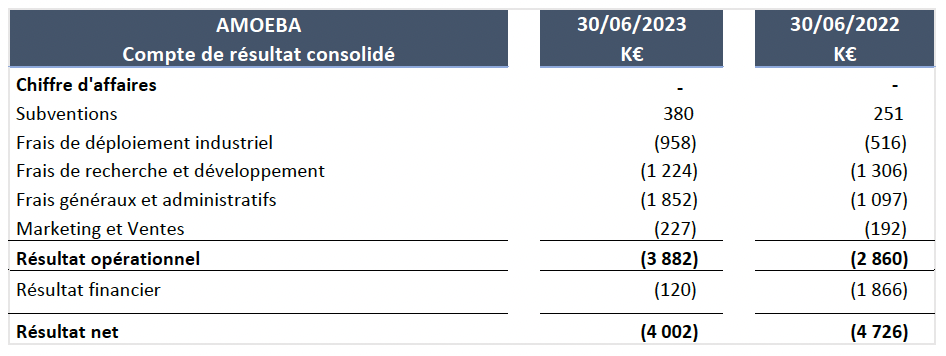
Operating income came to –€3,882K at June 30, 2023, compared with –€2,860K for the first half of 2022. No significant depreciation was recorded during the periods presented.
- The Company did not generate any revenues during the first half of 2023.
- The industrial deployment costs are €443K higher than the previous year, with this increase linked mainly to the transfer of consumable purchases and energy costs from the R&D department to the industrial deployment department.
- Research and development costs net of grants represent €1,224K, compared with €1,306K for the first half of 2022.
- Sales and marketing costs are up 18% from the previous year (€227K at June 30, 2023, versus €192K at June 30, 2022).
- Administrative costs and overheads increased by €755k compared with the previous year. This difference is mainly due to fees to secure financing and staff costs.
- The financial result is no longer impacted by bank loans, which have all been repaid.
- Half-year net income came to –€4,002K, compared with –€4,726K for the first half of 2022.
The Company’s cash position at June 30, 2023 was €3,136K, compared with €5,528K at December 31, 2022.
The reduction in the cash position reflects:
- cash flow from operating activities for –€2,221K;
- cash flow from investing activities for -€1,101K;
- cash flow from financing activities for +€927K.
At June 30, 2023, the Company’s shareholders’ equity represented €6.7m, compared with €8.2m at December 31, 2022.
The Company’s financial debt totaled €667K, comprising liabilities relating to lease obligations (€667K), as the bonds convertible into shares with an incentive program (OCAPI) had all been converted.
First half of the year marked primarily by the continued development of the biocontrol application and the new financing obtained
During the first half of 2023, Amoéba focused on the following main areas:
Industrial development:
On February 16, 2023, the Company announced that it had submitted the building permit application for its dedicated new production site for biocontrol applications, which will be located in Cavaillon in France’s Vaucluse region.
In line with the project’s schedule, the building permit application, setting out robust commitments to respect biodiversity and employees, was approved on June 12, 2023, following a three-month review. With this project, Amoéba aims to develop a dedicated industrial site of over 3,000 sq.m for the bio-production of its biocontrol agent. This major project is part of the industrialization plan launched by Amoéba in preparation for the commercialization of its plant protection products expected for early 2025.
Continuing to develop the biocontrol application:
- Collaboration with Nissan Chemical Corporation
On March 31, 2023, Amoéba announced that, under a material transfer agreement, Nissan Chemical Corporation has launched a study to assess the performance of a mixture combining one of its products with an experimental biocontrol product from Amoéba.
- Creation of the AXPERA brand
As part of its pre-commercialization plan, Amoéba has developed an international brand for its biocontrol solution, reflecting the values of innovation, performance, responsibility and renewal. On June 5, 2023, it announced that the brand name for its biocontrol solutions would be AXPERA, with its release planned for early 2025.
Field tests continuing to make progress The objectives of the tests are primarily to:
- Assess AXPERA’s efficacy and selectivity in the field on new targets (e.g. Arboriculture, Aromatic and ornamental plants, Strawberry, Garlic)
- Confirm AXPERA’s efficacy and selectivity on mildew and Uncinula necator or powdery mildew affecting grapes.
- Position the product within leading local agricultural programs in Europe.
- Assess AXPERA’s efficacy and selectivity with field tests for Soybean Rust in Brazil and Black Sigatoka affecting Bananas.
- Consolidate our efficacy data to further strengthen the applications for approval of the formulated product.
Since 2019 and at the date of the half-year report, the Company has carried out 620 tests, including 200 partner tests. It will continue with its tests in 2023.
Financing
- The Company announced the cancellation of the eighth optional tranche of 80 convertible bonds and the signing of a support contract with Redbridge Debt and Treasury Advisory, which aims to support the Company with adapting its financial strategy to its new industrial transformation challenges.
- Alongside this, Amoéba signed a new €9 million simple bond financing agreement with Nice & Green (see press release from February 15, 2023) with a view to launching its industrial project immediately.
- Amoéba also obtained €5.9m of funding from BPI France under the France 2030 program following its application for the “Agrifood Capacity and Resilience” call for projects. BPI France announced €5,917,676 of support based on a €3,550,606 grant and a €2,367,070 recoverable advance.
Coverage of the Company’s share
- The Company retained the Edison Group as an analyst to carry out a study into initiating financial coverage. This research contract, financed by the Company, aims to enable investors to broaden their understanding of the Company, which is necessary to take up positions on Amoéba shares (ALMIB).
- On April 20, Amoéba also welcomed Portzamparc – BNP Paribas Group on board to start covering its share. It began its monitoring with a “Buy” recommendation and a study entitled: “Amoeba supporting the environment”.
Governance
- At their Combined General Meeting on May 25, 2023, the Company’s shareholders decided to: renew the terms of office of Mr Plasson, Mr Dujardin, Mr Ambollet, Ms Guinard and Ms Filiatre, which had expired, for a six-year period through to the end of the Ordinary Annual General Meeting held in 2029 to approve the accounts for the year ending December 31, 2028.
- appoint Mr Jean Luc Souche (previously Biocontrol Business Developer with Amoéba) as a new director.
- The Board of Directors, which held its first session following the General Meeting, reappointed Mr Fabrice Plasson as Chairman of the Board of Directors and Chief Executive Officer for the duration of his term of office as a director.
- Valérie Filiatre, who opted to retire this year, will continue to support Amoéba’s development as a director. Jean-François DOUCET, who joined the company in May 2023, is now Deputy Managing Director.
“I would like to sincerely thank Valérie for her work with the company since 2014 and her commitment working alongside me during all these years. I wish her every happiness in her new projects, and I am delighted that she will continue to support the company as a director”, confirms Fabrice Plasson, Chairman and CEO.
Events since 30 June
On the biocontrol application
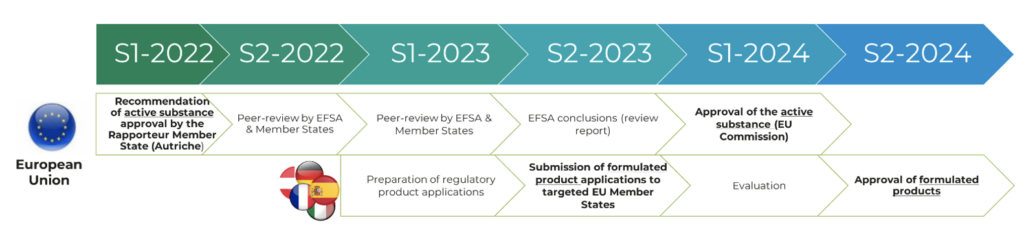
Amoéba confirms that the regulatory timetable for applying for approval of the biocontrol active substance is in line with the company’s forecasts (see press release from July 13, 2023).
Financing
The Company has drawn down the 1st tranche of 100 straight bonds subscribed to in favour of Nice & Green SA for a net amount of €2,820,000 paid in July 2023 and August 2023.
The Company is continuing its search for additional financing. Following an initial financial diagnosis and structuring phase carried out by Redbridge Debt and Treasury Advisory, the Company is continuing its fund-raising strategy with KPMG.
Investments
The Company confirms the progress of its project for a production site dedicated to biocontrol applications and announces that the deed of purchase for the land to house this plant in Cavaillon was signed on September 26, 2023.
Development and outlook
Biocontrol application
The Company is moving forward with its project to develop its first biocontrol plant. This plant, which will be located in Cavaillon in the South of France, is expected to be operational during the first quarter 2025 to align with sales of the biocontrol products.
Biocide application
Following the US EPA’s preliminary favorable decision on the use of the amoeba Willaertia Magna C2c Maky in closed cooling systems (see press release from August 10, 2022), Amoéba has started to look for a partner that will be able to take on the production and/or marketing of its biocide product in the United States. This search is still ongoing.
On the reporting date for the accounts, the Company has sufficient net working capital to cover its obligations and cash requirements through to June 30, 2024, and believes that it is in a position to deliver on the commitments that it has made to date.
Read the press release in PDF :
AMOEBA: Regulatory timetable for Europe’s biocontrol application on track
Chassieu (France), July 13th, 2023 – 08h30 – AMOÉBA (FR0011051598 – ALMIB) an industrial biotech in pre-commercialization* specialized in the treatment of microbiological risk, developing a biocontrol agent for the treatment of plants in agriculture and a biological biocide for the treatment of industrial water, confirms that the regulatory timetable for the biocontrol active substance application is in line with the Company’s forecasts.
The EFSA, the European Food Safety Agency, mentions on its website a Risk Assessment Deadline on July 19, 2023. As this date was assigned by default when the peer-review of dossier was initiated in December 2022, it does not take into account any delay by the authorities.
The Company had anticipated this possible delay in its forecasts: the regulatory timetable for its biocontrol applications in Europe, as published in the Universal Registration Document 2022 filed with the AMF on April 18, 2023 under the number D23-0296 and available on the Company’s website, is still valid and is recalled below:
- Risk assessment phase ending with EFSA’s peer-review report containing its conclusions: expected to be finalized in the second half of 2023.
- Risk management phase ending with the publication of the implementing regulation containing the European Commission’s decision in the Official Journal of the EU: expected to be finalized in the first half of 2024.
As applications for approval of formulated products can be submitted before definitive approval of the active substance, the forecast timetable for formulated products is also in line with the Company’s forecasts.
AMOEBA introduces its AXPERA brand for the biocontrol application
Chassieu (France), June 5th, 2023 – 17h45 – AMOÉBA (FR0011051598 – ALMIB) an industrial biotech in pre-commercialization* specialized in the treatment of microbiological risk, developing a biocontrol agent for the treatment of plants in agriculture and a biological biocide for the treatment of industrial water, announces today the brand name of its biocontrol solutions, with a view to commercialization expected in early 2025.

As part of the pre-commercialization plan, Amoéba has worked on the creation of an international brand for its biocontrol solution, reflecting the values of innovation, performance, responsibility and renewal.
Through its new brand AXPERA and its botanical design, Amoéba highlights its expertise in the service of a new “era” of plant protection. Amoéba keeps in the brand name the AXP prefix (Amoéba Experimental Product), as a reminder of the different experimental formulations (AXP12, AXP13, AXP17), part of its company history. The AXPERA brand may be declined in a range of products (AXPERA Joy, AXPERA Eva, AXPERA Noa…) in accordance with the company’s marketing positioning.
” Following the BIOMEBA brand for the biocide application, we are further concretising our biocontrol project with this new brand AXPERA. At the end of the creative process, we are delighted to present a brand name with a distinctive identity and a strong message of commitment. As we approach the launch of our product and its first commercialization, we will accelerate the notoriety of this brand, in particular on professional exhibitions and among the main actors of the industry” explains Anaïs LELLU, Communication Manager at Amoéba.
Since April 4, 2023, the AXPERA brand has been registered at the French National Institute of Industrial Property (INPI), under the N° 4951169 in class 05 Fungicide for agricultural use.
More information can be found on amoeba-nature.com, “Biocontrol“.
AMOEBA announces the initiation of coverage by Portzamparc – BNP Paribas Group
Chassieu (France), April 20, 2023 – 17h45 – AMOÉBA (FR0011051598 – ALMIB) an industrial biotech in pre-commercialization* specialized in the treatment of microbiological risk, developing a biocontrol agent for the treatment of plants in agriculture and a biological biocide for the treatment of industrial water, announces today that Portzamparc – BNP Paribas Group has initiated coverage on the company.
Portzamparc – BNP Paribas Group began its monitoring with a “Buy” recommendation and a study entitled: “the amoeba at the service of the environment”.Portzamparc ‘s study is available on Amoéba’s website in the “Shareholding / Coverage” section.
Ordinary and extraordinary general meeting to be help on 25 May 2023
MODALITIES THROUGH WHICH THE PREPARATORY DOCUMENTS WILL BE MADE AVAILABLE
Chassieu, 19 april 2023
The company’s shareholders are invited to attend the ordinary and extraordinary general meeting which will be held on:
Thursday 25 may 2023 – 9 am
at the company registered office – 38 Avenue des Frères Montgolfier- 69680 CHASSIEU
The notice of the meeting as a notice of convocation, including the agenda and the planned resolutions, was published in the BALO (Bulletin of obligatory legal announcements) of 19 april, 2023.
The documents stipulated by article R.225-83 of the French commercial code are available to shareholders from the moment that the assembly is convened, in line with the applicable regulatory requirements:
- Any registered shareholder can, until the fifth day (inclusive) before the assembly, ask the company to send him these documents. For holders of bearer shares, this right can only be exercised upon presentation of a statement of participation in the accounts of bearer shares held by the authorised intermediary;
- Any shareholder can consult these documents at the company’s headquarters during the 15 days preceding the date of the meeting.
For information, electronic voting via the VOTACCESS secure voting platform for the General Meeting of Thursday, May 25, 2023 will be open from May 3, 2023 until Wednesday, May 24 2023 at 3:00 pm (Paris time). Shareholders wishing to use this platform can consult the access conditions in the notice of meeting and on the company’s website (https://amoeba-nature.com/investisseur/assemblee-generale).
AMOÉBA announces the availability of the 2022 Universal Registration Document including the Annual Financial Report
Lyon (France), April 18, 2023 -5.45 pm- AMOÉBA (FR0011051598 – ALMIB), an industrial biotech in pre-commercialization* specialized in the treatment of microbiological risk, developing a biocontrol agent for the treatment of plants in agriculture and a biological biocide for the treatment of industrial water , announces that the 2022 Universal Registration Document was filed with the French Financial Markets Authority (Autorité des marchés financiers-AMF) on April 18, 2023 under number D.23-0296.
The 2022 Universal Registration Document includes:
- the Annual Financial Report comprising the consolidated financial statements, the parent company financial statements, the management report and the related statutory auditors’ reports
- The Board of Directors’ corporate governance report
The Universal Registration Document 2022 also presents the company’s activities, particularly in the development of the biocidal and biocontrol applications and its research works. The organisation, financial situation, results and prospects of Amoéba are also described in the Document.
The document may be viewed or downloaded on the company’s website www.amoeba-nature.com under section “Investors / financial documents/ Reference Document”. Copies of the Universal Registration Document are also available at the company’s headquarters: 38, avenue des frères Montgolfier, 69680 Chassieu, France.
Next event: General Meeting of Shareholders: 25 May 2023
Amoéba announces its collaboration with Nissan Chemical Corporation for the biocontrol application.
Lyon (France), March 31st, 2023– 08h30 – AMOÉBA (FR0011051598 – ALMIB) an industrial biotech in pre-commercialization* specialized in the treatment of microbiological risk, developing a biocontrol agent for the treatment of plants in agriculture and a biological biocide for the treatment of industrial water, announces that, in the framework of a Material Transfer Agreement, Nissan Chemical Corporation has started a performance evaluation study of the mixture of one of its products with an Amoéba biocontrol experimental product.
The aim of this evaluation is to determine the performance in controlling grapevine downy mildew of the combination of Nissan Leimay® product (suspension concentrate at 200 g/l amisulbrom) and Amoéba AXP12 experimental product (suspension concentrate at 215 g/l lysate of Willaertia magna C2c Maky),
This extemporaneous mixture was tested in two trials against grapevine downy mildew in Italy, in conditions of high disease pressure. Ten applications at 7-day intervals were made.
The combination Leimay®+ AXP12, tested at a reduced dose of both products, was very efficient on leaves and bunches. It is superior to Leimay® and AXP12 used alone at their full dose, which could allow a reduction in the dose of either or both products.
Moreover, the performance of this mixture is significantly better than the reference product of the trials, copper hydroxide (at the rate of 200 g active ingredient/ha/treatment).
These results support the continuation of the experiment in 2023.
Amoéba : 2022 annual results – An exceptional year which confirms Amoéba’s potential
Obtention of two marketing approvals in the USA (biocide and plant protection) and a recommendation for approval of the biocontrol active substance in Europe
Chassieu (France), March 30th, 2023 – 17:45 – AMOÉBA (FR0011051598 – ALMIB), an industrial biotech in pre-commercialization* specializing in the treatment of microbiological risk, developing a biocontrol agent for the treatment of plants in agriculture and a biological biocide for the treatment of industrial water, today announces its annual results for the year ending 31 December 2022.
The Board of Directors, which met on 30 March 2023, approved the corporate and consolidated financial statements of the Amoeba Group for the year ended 31 December 2022.
The Statutory Auditors have carried out their audit work and have not identified any significant anomalies that would call into question the conformity of the financial statements. The certification reports are being issued
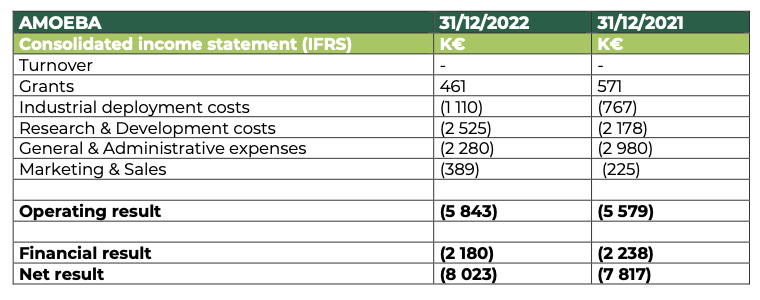
A financial position strongly improved by debt repayment and equity rebuilding
At 31 December 2022, Amoéba’s operating profit was -5,843 K€ compared to -5,579 K€ at 31 December 2021
- The company did not generate any revenue in the financial year 2022.
- Subsidies are down compared to 2021. They are mainly made up of the Research Tax Credit and underline the continued efforts made by the company in terms of research and development on its biocontrol application during 2022.
- Industrial deployment costs are up on the previous year and reflect the start of pre-industrialization of the biocontrol product developed by the Company.
- Research and Development expenses amount to €2,525K, up from €2,172K in 2021. This increase is mainly due to the external services provided to validate the field trials of the biocontrol product.
- General and administrative expenses amounted to €2,280k, down by €700k on the previous year.
- The increase in marketing and sales costs is mainly due to the strengthening of the team for pre-commercialization.
- The financial result mainly includes interest charges related to bank loans (€1,027k) and the OCAPI transaction (€1,153k).
The net result thus amounts to -€8,023k compared to -€7,817k at 31 December 2021.
At 31 December 2022, the company’s shareholders’ equity amounted to €8.2m compared with €0.2m at 31 December 2021.
The company’s financial debt amounted to €2.4m (compared to €12.5m at 31 December 2021) and is mainly made up of the bond loan resulting from bonds issued and not yet converted at the end of the year (€2.1m) and debts linked to lease obligations (€0.3m).
The Company’s cash position at 31 December 2022 was €5,528k compared with €7,275k at 31 December 2021.
An exceptional year marked by two marketing approvals in the USA (biocide and plant protection), the recommendation for approval of the biocontrol active substance in Europe and further development of pre-commercial field tests
During the year 2022, Amoéba focused on the following main areas:
1 – Biocontrol application
Sustainability of the company through regulatory announcements:
- On 25 April 2022, the Company announced that AGES (Agentur für Gesundheit und Ernährungssicherheit ), the competent authority of the Reporting Member State (Austria) in charge of the application for the biocontrol active substance “Willaertia magna C2c Maky Lysate”, recommends its approval for plant protection use in the European territory.
- In its draft assessment report, AGES concludes that the active substance is likely to meet the approval criteria. The Austrian authority thus confirms the efficacy of the active substance and its lack of adverse effects on human health and the environment when used according to good plant protection practice and under realistic conditions of use.
- On 29 September 2022, the company received a positive pre-decision from the US Environmental Protection Agency (US EPA) following the evaluation of the application for Willaertia magna C2c Maky as a biocontrol active substance (biopesticide) for use in agriculture. The EPA concluded that Willaertia magna C2c Makylysate has a low toxicity profile for human health and the environment, and that “its mode of action contributes to its attractiveness as a viable alternative to conventional pesticides, making it a valuable addition to the pesticide panel. Therefore, EPA is proposing to grant unconditional registration of Willaertia magna C2c Maky lysate as a new active ingredient in food and non-food plant protection uses.
- On 12 October 2022, following its favourable pre-decision, the EPA approved the maximum residue limit exemption (tolerance exemption) of Willaertia magna C2c Maky amoeba lysate, corresponding to the regulatory threshold of pesticide residue concentration, above which the marketing of a food product is no longer authorized.
- On 3 November 2022, the Company informed that the US Environmental Protection Agency (US EPA) has approved the use of the amoeba lysate Willaertia magna C2c Maky as an active substance for use in plant protection.
Recognition of its scientific expertise:
- On 18 October 2022, the Company announced the publication of a peer-reviewed scientific paper on its biocontrol application (https://www.mdpi.com/2223-7747/11/20/2756/pdf ) in the special “Plant Bioprotection” issue of Plants, the journal of the MDPI Group.
This article presents, for the first time to the international scientific community, the efficacy of Amoeba’s biocontrol products based on the lysate of the amoeba Willaertia magna C2c Maky on potato late blight.
The scientific data demonstrating the double mode of action of the lysate to control potato late blight are presented:
– On the indirect effect via the stimulation of the plant’s natural defences
– On the direct fungicidal effect against the pathogen Phytophtora infestans responsible for potato late blight.
An acceleration of field trials, synonymous with a record year:
- On 20 October 2022, the company announced the results of the field trial campaign.
With more than 120 field trials completed or underway in Europe, the United States, Brazil, Costa Rica and Asia, the winter 2021/summer 2022 field trial campaign is the largest ever undertaken by Amoéba.
The main objectives of these trials conducted by independent external service providers in small plots under GPE (Good Experimental Practice) guidelines were:
- To generate efficacy data for future marketing authorization applications (MA) in Europe, Brazil and California
- To evaluate the company’s formulations on new targets, in particular diseases of tropical crops, apples and certain vegetable crops.
To conduct parallel positioning trials in combination or in programs with other fungicides (notably on vines, potatoes, wheat, vegetables and soybeans), foreshadowing experiments closer to practical application.
2 – Biocide application
A strategic repositioning following regulatory announcements:
- On 3 May 2022, the Company announced that the MCCAA (Malta Competition and Consumer Affairs Authority), the competent authority of the reporting Member State (Malta) evaluating the application for approval of the biocidal active substance “Willaertia magna C2c Maky“, recommended its non-approval for biocidal use in cooling towers in Europe. On the basis of the application dossier for approval of the biocidal active substance “Willaertia magna C2c Maky“, the Maltese authority concluded in its draft report that the active substance is not likely to meet the approval criteria, considering that the innate efficacy has not been sufficiently demonstrated and that a Trojan horse effect cannot be excluded under realistic conditions of use.
- On 10 August 2022, the Company announced that the US Environmental Protection Agency (US EPA) has issued a favourable pre-decision following the evaluation of the application for authorization of Willaertia magna C2c Maky as a biocidal active substance in cooling systems.
As a result of this favourable EPA pre-decision and the public consultation required for any new active substance, completed on 3 December 2022, the amoeba Willaertia magna C2c Maky and BIOMEBA products containing it are now authorized in the United States for biocidal use in closed cooling systems, for the control of microbial slime, for the control of microbial induced corrosion and for the control of general microbial life.
- On October 19 2022, the Company announced that it would not pursue its live amoeba biocide application in Canada. Amoeba has decided to permanently withdraw the application for registration in Canada and to focus its resources on low regulatory risk applications.
3 –Securing the Company’s financing
- The Company announced the issuance of the fourth, fifth and sixth tranches of 60 convertible bonds and a seventh tranche of 40 convertible bonds as part of its incentive bond financing with Nice & Green.
- In addition, Amoéba finalized the restructuring of its debt by prepaying the entire EIB loan.
Developments and prospects
1 – Biocontrol application
The company is preparing to carry out a new field test campaign for its biocontrol product in 2023.
The program planned for 2023 will focus on the following themes:
– Completing and generating data for future marketing authorization dossiers in Europe and California
– Confirm in the second year the new strong points identified in 2022 (soybean rust, banana Sigatoka, apple scab, etc.)
– In vines and vegetables in Europe, test the inclusion of the Amoeba product in practical treatment programs: either in small plot trials under Good Experimental Practice or under real agronomic conditions with the farmer’s equipment
In Europe, the collective peer review of Austria’s draft report is underway and should be finalized in Q3 2023.
This should be followed by two further phases:
| Publication of the review report by the European Commission and implementing regulation carrying the European Commission’s decision | S1 2024 |
| Decisions on authorizations of products containing the active substance “Willaertia magna C2c Maky lysate” by targeted Member States | 2024 |
Applications for authorization of biocontrol products will be submitted in 2023 in the targeted member states: submission is indeed possible before the active substance is approved by the European Commission.
In the US, an application for approval of biocontrol products is expected to be submitted, following the approval of the active substance by the US EPA in 2022.
2 – USIBIAM industrialization project and search for financing
On January 27, 2023, the Company announced that it had terminated its convertible bond contract with Nice & Green SA and waived the issuance of the optional 8th tranche of 80 OCAs as initially foreseen in the contract[i].
Amoeba’s ambition is to build « USIBIAM » a production plant dedicated to biocontrol products, which could initially produce 40 tones of active substance per year, i.e. 200 tones of finished product, making it possible to treat 100,000 hectares. The company could increase its production and cover up to 200,000 hectares if the site is expanded. This production site should be operational by early 2025 in order to start marketing its biocontrol products as soon as marketing authorizations are obtained in Europe and the United States.
In order to finance this production site and to continue its operational and research activities over the next 3 years, the company estimates its total financial needs at €45 million – without extension-(€23 million in capital expenditure and €22 million in operational expenditure).
In order to adapt its financial strategy to its new industrial transformation challenges, Amoéba has concluded a support contract with Redbridge Debt and Treasury Advisory to assist it in its search for financing[ii].
Pending the search for and obtaining of such financing, on February 15, 2023, Nice & Green SA[iii] agreed to continue supporting Amoéba within the framework of a debt financing in the form of a simple bond loan with, as an exclusive repayment guarantee, a commitment to issue share warrants in the event of Amoéba’s failure to repay the simple bonds (OS) at their maturity. This interim financing of €9 millions allows the company to immediately start its industrial project and to cover its expenses until December 2023. It is intended to be automatically repaid as soon as Redbridge Debt and Treasury Advisory has structured a financial contribution.
On 16 February 2023, the Company announced that it had submitted the building permit for its new production site dedicated to biocontrol applications, based in Cavaillon in the Vaucluse.
On 29 March 2023, Amoéba announced its nomination as a winner of the France 2030 project following its application to the “Resilience and Agri-food Capacity” call for projects. After examining the application, BPI France recognized the quality and interest of Amoéba’s investments, a key player in the agro-ecological transition, and announced support of €5,917,676 in the form of a €3,550,606 grant and €2,367,070 in recoverable advance.
3 – Biocide application
Following the US EPA’s preliminary favourable decision on the use of the amoeba Willaertia Magna C2c Maky in closed cooling systems (see press release of 10 August 2022), Amoéba initiated a search in 2023 for a partner capable of taking over the production and/or marketing of the biocide product in the United States. This search is still ongoing.
4 – New applications
In addition to existing applications (biocide and biocontrol), Amoéba receives numerous requests to integrate its solution into new fields of operations. A strict scientific evaluation of these opportunities is carried out permanently by our laboratory and external expert laboratories.
At the date of closing the accounts, the Company has sufficient net working capital to meet its obligations and cash requirements over twelve months, believing that it can meet its commitments until December 2023. The financial statements for the year ended 31 December 2022 were approved by the Board of Directors on 30 March 2023 on a going concern basis in light of the business and cash flow forecasts.
Next event: General Meeting of Shareholders: 25 May 2023
[i] See press release of 21 december 2020
[ii] See press release of 27 january 2023
[iii] As a reminder, Nice & Green SA is an unregulated service provider and investor specialising in the structuring and financing of small and medium-sized listed companies, investing in particular in the medical science and technology sectors.
Amoéba obtains €5.9 million in funding from BPI France as part of France 2030 program
Chassieu (France), March 29th, 2023 – 17:45 – AMOÉBA (FR0011051598 – ALMIB), an industrial biotech in pre-commercialization* specializing in the treatment of microbiological risk, developing a biocontrol agent for the treatment of plants in agriculture and a biological biocide for the treatment of industrial water, announces the support of BPI France in the framework of the national financing plan “France 2030”.
Presented in October 2021, the “France 2030” financing plan aims to provide answers to the major challenges of our time, while raising France to the rank of leader in its industry through three levers: reindustrialising France, investing massively in innovative technologies and supporting the ecological transition.
As a result of its application to the « Résilience et Capacité Agroalimentaire » appeal, Amoéba has officially been nominated as the laureate of the France 2030 project. After examining the application, BPI France recognised the quality and interest of Amoéba’s investments, as it is a key player in the agro-ecological transition, and announced a support of €5,917,676 in the form of a €3,550,606 subsidy and a €2,367,070 recoverable advance. At this stage, the company is still pending the terms and timing of the payment of these amounts.
This financing will enable the company to further its industrial project, in particular the creation of its eco-responsible plant, located in the new business park “Hauts Banquets” in Cavaillon, dedicated to naturalness. In addition to the environmental nature of the project, Amoéba’s industrialisation meets the innovation criteria of France 2030, with 4.0 facilities and the use of artificial intelligence solutions.
As a reminder, in order to finance its future production site and pursue its operational and research activities over the next three years, the company estimates its total financial expenditure at 45 million euros. Amoéba is currently pursuing its research for the required financing(s).
By industrializing the production of biocontrol solutions in France, Amoéba is committed to putting all its efforts into biological, autonomous and safe agriculture, and to meeting the European objectives of a 50% reduction in the use of pesticides in Europe by 2030, a 25% of cultures treated in Organic Agriculture in Europe by 2030 and the securing of food inputs in Europe.
NEXT APPOINTMENT : March 30th, 2023 – 2022 Annual results
This project was supported by France 2030
AMOEBA announces initiation report from the analyst Edison Group
Lyon (France), March 13th, 2023 – 17:45 – AMOÉBA (FR0011051598 – ALMIB), an industrial biotech in pre-commercialization* specializing in the treatment of microbiological risk, developing a biocontrol agent for the treatment of plants in agriculture and a biological biocide for the treatment of industrial water, announces that Edison Group, a London-based equity research house, has initiated coverage on the company.
This research contract, financed by the company, aims to allow investors to broaden their understanding of the company, which is necessary to take a position on Amoéba shares (ALMIB).
The first note, entitled “Amoéba, fighting pathogens, respecting nature” is available to all investors and provides insight into the business’ plans to raise €45m to fund its operations and expand its site over the next three years. Subsequent research will keep shareholders up to date with Amoeba’s finances, strategy and operational progress.
The company informs that, as Amoéba plans to double the size of its biocontrol plant in Cavaillon in 2027, Edison Group’s estimations on this first note are based on a production capacity allowing the treatment of 200 000 hectares.
Edison’s research is available on Amoéba’s website in the “Shareholding / Coverage” section as well as directly from the Edison website and via major financial data providers including Bloomberg.
