Amoéba : 2022 results
Amoéba : 2022 annual results – An exceptional year which confirms Amoéba’s potential
Obtention of two marketing approvals in the USA (biocide and plant protection) and a recommendation for approval of the biocontrol active substance in Europe
Chassieu (France), March 30th, 2023 – 17:45 – AMOÉBA (FR0011051598 – ALMIB), an industrial biotech in pre-commercialization* specializing in the treatment of microbiological risk, developing a biocontrol agent for the treatment of plants in agriculture and a biological biocide for the treatment of industrial water, today announces its annual results for the year ending 31 December 2022.
The Board of Directors, which met on 30 March 2023, approved the corporate and consolidated financial statements of the Amoeba Group for the year ended 31 December 2022.
The Statutory Auditors have carried out their audit work and have not identified any significant anomalies that would call into question the conformity of the financial statements. The certification reports are being issued
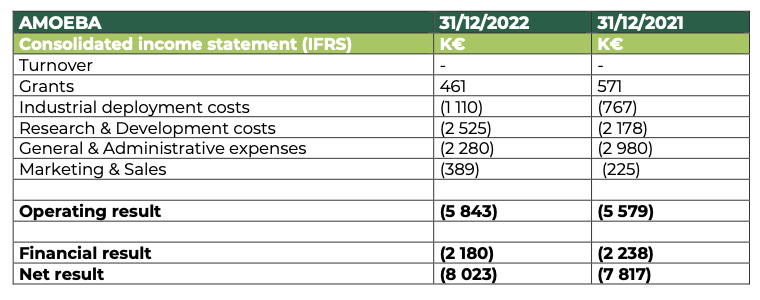
A financial position strongly improved by debt repayment and equity rebuilding
At 31 December 2022, Amoéba’s operating profit was -5,843 K€ compared to -5,579 K€ at 31 December 2021
- The company did not generate any revenue in the financial year 2022.
- Subsidies are down compared to 2021. They are mainly made up of the Research Tax Credit and underline the continued efforts made by the company in terms of research and development on its biocontrol application during 2022.
- Industrial deployment costs are up on the previous year and reflect the start of pre-industrialization of the biocontrol product developed by the Company.
- Research and Development expenses amount to €2,525K, up from €2,172K in 2021. This increase is mainly due to the external services provided to validate the field trials of the biocontrol product.
- General and administrative expenses amounted to €2,280k, down by €700k on the previous year.
- The increase in marketing and sales costs is mainly due to the strengthening of the team for pre-commercialization.
- The financial result mainly includes interest charges related to bank loans (€1,027k) and the OCAPI transaction (€1,153k).
The net result thus amounts to -€8,023k compared to -€7,817k at 31 December 2021.
At 31 December 2022, the company’s shareholders’ equity amounted to €8.2m compared with €0.2m at 31 December 2021.
The company’s financial debt amounted to €2.4m (compared to €12.5m at 31 December 2021) and is mainly made up of the bond loan resulting from bonds issued and not yet converted at the end of the year (€2.1m) and debts linked to lease obligations (€0.3m).
The Company’s cash position at 31 December 2022 was €5,528k compared with €7,275k at 31 December 2021.
An exceptional year marked by two marketing approvals in the USA (biocide and plant protection), the recommendation for approval of the biocontrol active substance in Europe and further development of pre-commercial field tests
During the year 2022, Amoéba focused on the following main areas:
1 – Biocontrol application
Sustainability of the company through regulatory announcements:
- On 25 April 2022, the Company announced that AGES (Agentur für Gesundheit und Ernährungssicherheit ), the competent authority of the Reporting Member State (Austria) in charge of the application for the biocontrol active substance “Willaertia magna C2c Maky Lysate”, recommends its approval for plant protection use in the European territory.
- In its draft assessment report, AGES concludes that the active substance is likely to meet the approval criteria. The Austrian authority thus confirms the efficacy of the active substance and its lack of adverse effects on human health and the environment when used according to good plant protection practice and under realistic conditions of use.
- On 29 September 2022, the company received a positive pre-decision from the US Environmental Protection Agency (US EPA) following the evaluation of the application for Willaertia magna C2c Maky as a biocontrol active substance (biopesticide) for use in agriculture. The EPA concluded that Willaertia magna C2c Makylysate has a low toxicity profile for human health and the environment, and that “its mode of action contributes to its attractiveness as a viable alternative to conventional pesticides, making it a valuable addition to the pesticide panel. Therefore, EPA is proposing to grant unconditional registration of Willaertia magna C2c Maky lysate as a new active ingredient in food and non-food plant protection uses.
- On 12 October 2022, following its favourable pre-decision, the EPA approved the maximum residue limit exemption (tolerance exemption) of Willaertia magna C2c Maky amoeba lysate, corresponding to the regulatory threshold of pesticide residue concentration, above which the marketing of a food product is no longer authorized.
- On 3 November 2022, the Company informed that the US Environmental Protection Agency (US EPA) has approved the use of the amoeba lysate Willaertia magna C2c Maky as an active substance for use in plant protection.
Recognition of its scientific expertise:
- On 18 October 2022, the Company announced the publication of a peer-reviewed scientific paper on its biocontrol application (https://www.mdpi.com/2223-7747/11/20/2756/pdf ) in the special “Plant Bioprotection” issue of Plants, the journal of the MDPI Group.
This article presents, for the first time to the international scientific community, the efficacy of Amoeba’s biocontrol products based on the lysate of the amoeba Willaertia magna C2c Maky on potato late blight.
The scientific data demonstrating the double mode of action of the lysate to control potato late blight are presented:
– On the indirect effect via the stimulation of the plant’s natural defences
– On the direct fungicidal effect against the pathogen Phytophtora infestans responsible for potato late blight.
An acceleration of field trials, synonymous with a record year:
- On 20 October 2022, the company announced the results of the field trial campaign.
With more than 120 field trials completed or underway in Europe, the United States, Brazil, Costa Rica and Asia, the winter 2021/summer 2022 field trial campaign is the largest ever undertaken by Amoéba.
The main objectives of these trials conducted by independent external service providers in small plots under GPE (Good Experimental Practice) guidelines were:
- To generate efficacy data for future marketing authorization applications (MA) in Europe, Brazil and California
- To evaluate the company’s formulations on new targets, in particular diseases of tropical crops, apples and certain vegetable crops.
To conduct parallel positioning trials in combination or in programs with other fungicides (notably on vines, potatoes, wheat, vegetables and soybeans), foreshadowing experiments closer to practical application.
2 – Biocide application
A strategic repositioning following regulatory announcements:
- On 3 May 2022, the Company announced that the MCCAA (Malta Competition and Consumer Affairs Authority), the competent authority of the reporting Member State (Malta) evaluating the application for approval of the biocidal active substance “Willaertia magna C2c Maky“, recommended its non-approval for biocidal use in cooling towers in Europe. On the basis of the application dossier for approval of the biocidal active substance “Willaertia magna C2c Maky“, the Maltese authority concluded in its draft report that the active substance is not likely to meet the approval criteria, considering that the innate efficacy has not been sufficiently demonstrated and that a Trojan horse effect cannot be excluded under realistic conditions of use.
- On 10 August 2022, the Company announced that the US Environmental Protection Agency (US EPA) has issued a favourable pre-decision following the evaluation of the application for authorization of Willaertia magna C2c Maky as a biocidal active substance in cooling systems.
As a result of this favourable EPA pre-decision and the public consultation required for any new active substance, completed on 3 December 2022, the amoeba Willaertia magna C2c Maky and BIOMEBA products containing it are now authorized in the United States for biocidal use in closed cooling systems, for the control of microbial slime, for the control of microbial induced corrosion and for the control of general microbial life.
- On October 19 2022, the Company announced that it would not pursue its live amoeba biocide application in Canada. Amoeba has decided to permanently withdraw the application for registration in Canada and to focus its resources on low regulatory risk applications.
3 –Securing the Company’s financing
- The Company announced the issuance of the fourth, fifth and sixth tranches of 60 convertible bonds and a seventh tranche of 40 convertible bonds as part of its incentive bond financing with Nice & Green.
- In addition, Amoéba finalized the restructuring of its debt by prepaying the entire EIB loan.
Developments and prospects
1 – Biocontrol application
The company is preparing to carry out a new field test campaign for its biocontrol product in 2023.
The program planned for 2023 will focus on the following themes:
– Completing and generating data for future marketing authorization dossiers in Europe and California
– Confirm in the second year the new strong points identified in 2022 (soybean rust, banana Sigatoka, apple scab, etc.)
– In vines and vegetables in Europe, test the inclusion of the Amoeba product in practical treatment programs: either in small plot trials under Good Experimental Practice or under real agronomic conditions with the farmer’s equipment
In Europe, the collective peer review of Austria’s draft report is underway and should be finalized in Q3 2023.
This should be followed by two further phases:
| Publication of the review report by the European Commission and implementing regulation carrying the European Commission’s decision | S1 2024 |
| Decisions on authorizations of products containing the active substance “Willaertia magna C2c Maky lysate” by targeted Member States | 2024 |
Applications for authorization of biocontrol products will be submitted in 2023 in the targeted member states: submission is indeed possible before the active substance is approved by the European Commission.
In the US, an application for approval of biocontrol products is expected to be submitted, following the approval of the active substance by the US EPA in 2022.
2 – USIBIAM industrialization project and search for financing
On January 27, 2023, the Company announced that it had terminated its convertible bond contract with Nice & Green SA and waived the issuance of the optional 8th tranche of 80 OCAs as initially foreseen in the contract[i].
Amoeba’s ambition is to build « USIBIAM » a production plant dedicated to biocontrol products, which could initially produce 40 tones of active substance per year, i.e. 200 tones of finished product, making it possible to treat 100,000 hectares. The company could increase its production and cover up to 200,000 hectares if the site is expanded. This production site should be operational by early 2025 in order to start marketing its biocontrol products as soon as marketing authorizations are obtained in Europe and the United States.
In order to finance this production site and to continue its operational and research activities over the next 3 years, the company estimates its total financial needs at €45 million – without extension-(€23 million in capital expenditure and €22 million in operational expenditure).
In order to adapt its financial strategy to its new industrial transformation challenges, Amoéba has concluded a support contract with Redbridge Debt and Treasury Advisory to assist it in its search for financing[ii].
Pending the search for and obtaining of such financing, on February 15, 2023, Nice & Green SA[iii] agreed to continue supporting Amoéba within the framework of a debt financing in the form of a simple bond loan with, as an exclusive repayment guarantee, a commitment to issue share warrants in the event of Amoéba’s failure to repay the simple bonds (OS) at their maturity. This interim financing of €9 millions allows the company to immediately start its industrial project and to cover its expenses until December 2023. It is intended to be automatically repaid as soon as Redbridge Debt and Treasury Advisory has structured a financial contribution.
On 16 February 2023, the Company announced that it had submitted the building permit for its new production site dedicated to biocontrol applications, based in Cavaillon in the Vaucluse.
On 29 March 2023, Amoéba announced its nomination as a winner of the France 2030 project following its application to the “Resilience and Agri-food Capacity” call for projects. After examining the application, BPI France recognized the quality and interest of Amoéba’s investments, a key player in the agro-ecological transition, and announced support of €5,917,676 in the form of a €3,550,606 grant and €2,367,070 in recoverable advance.
3 – Biocide application
Following the US EPA’s preliminary favourable decision on the use of the amoeba Willaertia Magna C2c Maky in closed cooling systems (see press release of 10 August 2022), Amoéba initiated a search in 2023 for a partner capable of taking over the production and/or marketing of the biocide product in the United States. This search is still ongoing.
4 – New applications
In addition to existing applications (biocide and biocontrol), Amoéba receives numerous requests to integrate its solution into new fields of operations. A strict scientific evaluation of these opportunities is carried out permanently by our laboratory and external expert laboratories.
At the date of closing the accounts, the Company has sufficient net working capital to meet its obligations and cash requirements over twelve months, believing that it can meet its commitments until December 2023. The financial statements for the year ended 31 December 2022 were approved by the Board of Directors on 30 March 2023 on a going concern basis in light of the business and cash flow forecasts.
Next event: General Meeting of Shareholders: 25 May 2023
[i] See press release of 21 december 2020
[ii] See press release of 27 january 2023
[iii] As a reminder, Nice & Green SA is an unregulated service provider and investor specialising in the structuring and financing of small and medium-sized listed companies, investing in particular in the medical science and technology sectors.
